Background and Significance: Anemia is a common and debilitating symptom in patients with myelodysplastic syndromes (MDS), frequently leading to dependence on red blood cell (RBC) transfusions. Transfusion-dependent (TD) patients have worse overall survival and quality of life, and repeat transfusions are associated with complications, including iron overload. Erythropoiesis-stimulating agents (ESAs) are the standard treatment for anemia in patients with lower-risk (LR)-MDS, but many patients are ineligible for or refractory to ESAs, and responses are limited.
Luspatercept, a late-stage erythroid maturation agent, is approved to treat TD patients with LR-MDS after ESA failure. In the phase 3 COMMANDS study, in ESA-naive patients with LR-MDS requiring transfusions, a significantly higher proportion of patients receiving luspatercept than epoetin alfa achieved RBC transfusion independence for ≥ 12 weeks with concurrent hemoglobin (Hb) increase ≥ 1.5 g/dL (weeks 1-24) (Platzbecker U, et al. Lancet 2023. doi: 10.1016/S0140-6736(23)00874-7). The potential of luspatercept to improve moderately severe chronic anemia and reduce RBC transfusion dependence has not yet been investigated in the majority non-transfusion-dependent (NTD) LR-MDS patient population.
Preventing transfusion dependence is crucial for improving LR-MDS patient outcomes. The aim of this actively enrolling, phase 3, randomized, multicenter study is to compare the safety and efficacy of luspatercept versus epoetin alfa in reducing progression to RBC transfusion dependence in ESA-naive, NTD adult patients with anemia due to LR-MDS.
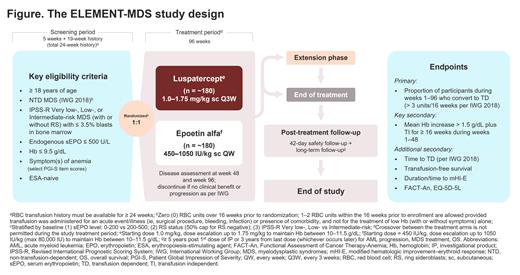
Study design and methods: The ELEMENT-MDS study includes a 5-week screening period, a 96-week treatment period, an extension phase, and a post-treatment follow-up period (Figure). Target enrollment is 360 patients with anemia due to Revised International Prognostic Scoring System (IPSS-R) Very low-, Low-, or Intermediate-risk MDS. Eligible patients are ≥ 18 years of age with a confirmed MDS diagnosis, baseline serum erythropoietin (sEPO) levels ≤ 500 U/L, and symptomatic anemia defined as a score of moderate or worse on ≥ 1 Patient Global Impression of Severity (PGI-S) item (fatigue, shortness of breath, weakness, or dizziness). Participants must be RBC transfusion-independent per International Working Group 2018 criteria (no RBC transfusions within 16 weeks prior to randomization) and have baseline Hb ≤ 9.5 g/dL. Patients will be excluded if they have a del(5q) cytogenetic abnormality, unclassifiable MDS or myelodysplastic/myeloproliferative neoplasms, anemia due to reasons other than MDS, bleeding disorders, low absolute neutrophil or platelet counts, uncontrolled hypertension, previous acute myeloid leukemia diagnosis, or history of recent malignancies, among other reasons.
Participants will be randomized 1:1 to receive luspatercept or epoetin alfa. Stratification factors include baseline sEPO levels, ring sideroblast status, and IPSS-R risk category. Patients will receive luspatercept once every 3 weeks at a starting dose of 1.0 mg/kg (escalation up to 1.75 mg/kg allowed) or epoetin alfa once a week at a starting dose of 450 IU/kg (escalation up to 1050 IU/kg allowed). The primary endpoint will compare the proportion of patients becoming TD (defined as requiring ≥ 3 RBC units/16 weeks) during any continuous 16-week interval in weeks 1-96. The key secondary endpoint is a Hb increase ≥ 1.5 g/dL sustained for ≥ 16 weeks during weeks 1-48. Additional secondary endpoints include time to RBC transfusion dependence, transfusion-free survival, time to achieve modified hematologic improvement-erythroid response, transfusion independence ≥ 24 weeks, and quality of life assessment. Safety endpoints include type and severity of adverse events and relationship to treatment, overall survival, and progression to high-risk MDS and acute myeloid leukemia.
Summary: The results of this phase 3 study will determine the efficacy and safety of luspatercept as a therapy for ESA-naive NTD patients with LR-MDS, who have limited treatment options for anemia. The study is registered at ClinicalTrials.gov (NCT05949684) and EudraCT (2022-500430-29-00).
Disclosures
Zeidan:Zentalis: Consultancy, Honoraria; Notable: Consultancy, Honoraria; Ionis: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; ALX Oncology: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Foran: Consultancy, Research Funding; Syros: Consultancy, Honoraria; Boehringer-Ingelheim: Consultancy, Honoraria; Chiesi: Consultancy, Honoraria; Mendus: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Otsuka: Consultancy, Honoraria; BeyondSpring: Consultancy, Honoraria; BioCryst: Consultancy, Honoraria; Orum: Consultancy, Honoraria; Shattuck Labs: Research Funding; Servier: Consultancy, Honoraria; Kura: Consultancy, Honoraria; Lox Oncology: Consultancy, Honoraria; Taiho: Consultancy, Honoraria; Geron: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Syndax: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Agios: Consultancy, Honoraria; Schrödinger: Consultancy, Honoraria; Regeneron: Consultancy, Honoraria; Tyme: Consultancy, Honoraria; Astex: Research Funding; Takeda: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria. Komrokji:Geron: Consultancy; Rigel, Taiho, DSI: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie, CTI biopharma, Jazz, Pharma Essentia, Servio: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees. Buckstein:Taiho: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Abbvie: Honoraria. Santini:AbbVie: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Geron: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; CTI: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Otsuka: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees; Janssen: Other: Travel support. Rose:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company; Celgene: Current holder of stock options in a privately-held company. Malini:Bristol Myers Squibb: Current Employment. Lew:Bristol Myers Squibb, Inc.: Current Employment, Current equity holder in publicly-traded company. Aggarwal:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Keeperman:Pfizer: Divested equity in a private or publicly-traded company in the past 24 months; Celgene: Divested equity in a private or publicly-traded company in the past 24 months; Bristol Myers Squibb: Current Employment. Jiang:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Giuseppi:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Zhang:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Cluzeau:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: support attending meetings/travel; Agios: Consultancy; Servier: Consultancy, Honoraria, Other: support attending meetings/travel; BluePrint: Consultancy; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: support attending meetings/travel; Astellas: Honoraria; Incyte: Honoraria; Pfizer: Other: support attending meetings/travel; Jazz Pharma: Consultancy, Honoraria; AbbVie: Consultancy, Other: support attending meetings/travel. Shortt:Amgen: Research Funding; Pfizer: Consultancy; Otsuka: Consultancy; Novartis: Consultancy, Speakers Bureau; Mundipharma: Consultancy, Speakers Bureau; Astellas: Consultancy; BMS: Consultancy, Research Funding. Platzbecker:Curis: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Jazz: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy; Janssen Biotech: Consultancy, Research Funding; Merck: Research Funding; BMS: Research Funding; BeiGene: Research Funding; Fibrogen: Research Funding; Syros: Consultancy, Honoraria, Research Funding; MDS Foundation: Membership on an entity's Board of Directors or advisory committees; Roche: Research Funding; Servier: Consultancy, Honoraria, Research Funding; Celgene: Honoraria; Silence Therapeutics: Consultancy, Honoraria, Research Funding; Geron: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support; medical writing support, Research Funding; Takeda: Consultancy, Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal